用戶:Zhantongz/沙盒1
現在正在的進行的沙盒1項目:分子理論史
| “ | 此項目在完成後將會立即移至分子理論史條目 | ” |

現代分子的概念可以追溯到古希臘哲學家留基伯時代,他認為宇宙是由原子和空洞組成。早在戰國時期,儒家經典《尚書‧洪範》曰:「五行,一曰水、二曰火、三曰木、四曰金、五曰土」[1],即五行。五行是中國最早的元素概念。在公元前約450年,古希臘人恩培多克勒想像出構成世界的是基本元素(火(![]() )、地(
)、地(![]() )、風(
)、風(![]() )和水(
)和水(![]() ))以及這些元素間的相互作用力。在此之前,赫拉克利特曾聲稱我們生存的根本是由相反屬性結合成的火。[2]在《蒂邁歐篇》中,受畢達哥拉斯影響的柏拉圖認為數學實體(例如數、點、線和三角形)是世界的基本構成元素,並認為火、地、風和水四種元素是由數學原理或元素能通過的東西組成的物質。[3] 第五種元素,以太,被認為是天體的基本構成物質。恩培多克勒和留基伯的觀點,與以太一起,被亞里士多德接受並傳遞到中世紀和文藝復興時期的歐洲。
))以及這些元素間的相互作用力。在此之前,赫拉克利特曾聲稱我們生存的根本是由相反屬性結合成的火。[2]在《蒂邁歐篇》中,受畢達哥拉斯影響的柏拉圖認為數學實體(例如數、點、線和三角形)是世界的基本構成元素,並認為火、地、風和水四種元素是由數學原理或元素能通過的東西組成的物質。[3] 第五種元素,以太,被認為是天體的基本構成物質。恩培多克勒和留基伯的觀點,與以太一起,被亞里士多德接受並傳遞到中世紀和文藝復興時期的歐洲。
隨着實驗證明了純化學元素(單質)的存在,並又有實驗發現了不同的單個原子形成化學性質穩定的分子(如氫氣和氧氣的單個原子可以結合起來形成水分子)的過程,現代概念上的分子在19世紀開始發展。
17世紀[編輯]
The earliest views on the shapes and connectivity of atoms was that proposed by Leucippus, Democritus, and Epicurus who reasoned that the solidness of the material corresponded to the shape of the atoms involved. Thus, iron atoms are solid and strong with hooks that lock them into a solid; water atoms are smooth and slippery; salt atoms, because of their taste, are sharp and pointed; and air atoms are light and whirling, pervading all other materials.[4] It was Democritus that was the main proponent of this view. Using analogies from our sense experiences, he gave a picture or an image of an atom in which atoms were distinguished from each other by their shape, their size, and the arrangement of their parts. Moreover, connections were explained by material links in which single atoms were supplied with attachments: some with hooks and eyes others with balls and sockets.[5]
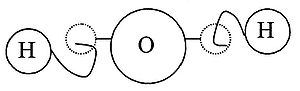
With the rise of Christianity and the decline of the Roman Empire, the atomic theory was abandoned for nearly two millennia in favor of the various four element theories and later alchemical theories. The 17th century, however, saw a resurgence in the atomic theory primarily through the works of Descartes, Gassendi, and Newton. Using earlier Greek atomic theories to explain how the tiniest particles of matter bonded together, Descartes visualized that atoms were held together by microscopic hooks and barbs.[6] He held that two atoms combined when the hook of one got caught in the eye of the other (see diagram):
By the mid 1770s, it was generally believed that any theory involving particles endowed with physical hooks was considered 「Cartesian chemistry」.[7] Similar to Descartes, Gassendi, who had recently written a book on the life of Epicurus, reasoned that to account for the size and shape of atoms moving in a void could account for the properties of matter. Heat was due to small, round atoms; cold, to pyramidal atoms with sharp points, which accounted for the pricking sensation of severe cold; and solids were held together by interlacing hooks.[8]
Newton, though he acknowledged the various atom attachment theories in vogue at the time, i.e. 「hooked atoms」, 「glued atoms」 (bodies at rest), and the 「stick together by conspiring motions」 theory, rather believed, as famously stated in "Query 31" of his 1704 Opticks, that particles attract one another by some force, which 「in immediate contact is extremely strong, at small distances performs the chemical operations, and reaches not far from particles with any sensible effect.」 [9]
In a more concrete manner, however, the concept of aggregates or units of bonded atoms, i.e. "molecules", traces its origins to Robert Boyle's 1661 hypothesis, in his famous treatise The Sceptical Chymist, that matter is composed of clusters of particles and that chemical change results from the rearrangement of the clusters. Boyle argued that matter's basic elements consisted of various sorts and sizes of particles, called "corpuscles", which were capable of arranging themselves into groups.
In 1680, using the corpuscular theory as a basis, French chemist Nicolas Lemery stipulated that the acidity of any substance consisted in its pointed particles, while alkalis were endowed with pores of various sizes.[10] A molecule, according to this view, consisted of corpuscles united through a geometric locking of points and pores.
18世紀[編輯]

An early precursor to the idea of bonded "combinations of atoms", was the theory of "combination via chemical affinity". For example, in 1718, building on Boyle’s conception of combinations of clusters, the French chemist Étienne François Geoffroy developed theories of chemical affinity to explain combinations of particles, reasoning that a certain alchemical 「force」 draws certain alchemical components together. Geoffroy's name is best known in connection with his tables of "affinities" (tables des rapports), which he presented to the French Academy in 1718 and 1720.
These were lists, prepared by collating observations on the actions of substances one upon another, showing the varying degrees of affinity exhibited by analogous bodies for different reagents. These tables retained their vogue for the rest of the century, until displaced by the profounder conceptions introduced by CL Berthollet.
In 1738, Swiss physicist and mathematician Daniel Bernoulli published Hydrodynamica, which laid the basis for the kinetic theory of gases. In this work, Bernoulli positioned the argument, still used to this day, that gases consist of great numbers of molecules moving in all directions, that their impact on a surface causes the gas pressure that we feel, and that what we experience as heat is simply the kinetic energy of their motion. The theory was not immediately accepted, in part because conservation of energy had not yet been established, and it was not obvious to physicists how the collisions between molecules could be perfectly elastic.
In 1789, William Higgins published views on what he called combinations of "ultimate" particles, which foreshadowed the concept of valency bonds. If, for example, according to Higgins, the force between the ultimate particle of oxygen and the ultimate particle of nitrogen were 6, then the strength of the force would be divided accordingly, and similarly for the other combinations of ultimate particles:

19世紀[編輯]

1803年,約翰·道爾頓以最輕的原子 - 氫原子的原子質量為單位,用比例來描述化合物。例如,亞硝酸酐的原子個數比為2:3給出的化學式為 N2O3。有趣的是,道爾頓錯誤地想像原子互相「鈎住」形成分子。
後來,1808年,道爾頓發表了著名的原子組合圖:

在阿莫迪歐·阿伏伽德羅1811年發表的 "Essay on Determining the Relative Masses of the Elementary Molecules of Bodies"論文中,他本質上指出:[11]
氣體的最小粒子是不一定是單個的原子,而是一些原子以一定的吸引力結合在一起形成的一個分子。
注意這句話並非直譯。阿伏伽德羅把原子和分子都稱為」分子「 。具體來說,他使用」初級分子「來表述原子,並使用」複合分子「和」超複合分子「來表達物質的聚合。
During his stay in Vercelli, Avogadro wrote a concise note (memoria) in which he declared the hypothesis of what we now call Avogadro's law: equal volumes of gases, at the same temperature and pressure, contain the same number of molecules. This law implies that the relationship occurring between the weights of same volumes of different gases, at the same temperature and pressure, corresponds to the relationship between respective molecular weights. Hence, relative molecular masses could now be calculated from the masses of gas samples.
Avogadro developed this hypothesis in order to reconcile Joseph Louis Gay-Lussac's 1808 law on volumes and combining gases with Dalton's 1803 atomic theory. The greatest difficulty Avogadro had to resolve was the huge confusion at that time regarding atoms and molecules—one of the most important contributions of Avogadro's work was clearly distinguishing one from the other, admitting that simple particles too could be composed of molecules, and that these are composed of atoms. Dalton, by contrast, did not consider this possibility. Curiously, Avogadro considers only molecules containing even numbers of atoms; he does not say why odd numbers are left out.
In 1826, building on the work of Avogadro, the French chemist Jean-Baptiste Dumas states:
Gases in similar circumstances are composed of molecules or atoms placed at the same distance, which is the same as saying that they contain the same number in the same volume.
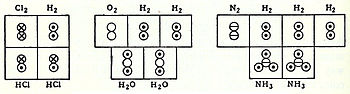
In coordination with these concepts, in 1833 the French chemist Marc Antoine Auguste Gaudin presented a clear account of Avogadro's hypothesis,[12] regarding atomic weights, by making use of 「volume diagrams」, which clearly show both semi-correct molecular geometries, such as a linear water molecule, and correct molecular formulas, such as H2O:
In two papers outlining his "theory of atomicity of the elements" (1857–58), Friedrich August Kekulé was the first to offer a theory of how every atom in an organic molecule was bonded to every other atom. He proposed that carbon atoms were tetravalent, and could bond to themselves to form the carbon skeletons of organic molecules.
In 1856, Scottish chemist Archibald Couper began research on the bromination of benzene at the laboratory of Charles Wurtz in Paris.[13] One month after Kekulé's second paper appeared, Couper's independent and largely identical theory of molecular structure was published. He offered a very concrete idea of molecular structure, proposing that atoms joined to each other like modern-day Tinkertoys in specific three-dimensional structures. Couper was the first to use lines between atoms, in conjunction with the older method of using brackets, to represent bonds, and also postulated straight chains of atoms as the structures of some molecules, ring-shaped molecules of others, such as in tartaric acid and cyanuric acid [14] In later publications, Couper’s bonds were represented using straight dotted lines (although it is not known if this is the typesetter’s preference) such as with alcohol and oxalic acid below:

In 1861, an unknown Vienna high-school teacher named Joseph Loschmidt published, at his own expense, a booklet entitled Chemische Studien I, containing pioneering molecular images which showed both "ringed" structures as well as double-bonded structures, such as:[15]
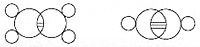
Loschmidt also suggested a possible formula for benzene, but left the issue open. The first proposal of the modern structure for benzene was due to Kekulé, in 1865. The cyclic nature of benzene was finally confirmed by the crystallographer Kathleen Lonsdale. Benzene presents a special problem in that, to account for all the bonds, there must be alternating double carbon bonds:

In 1865, German chemist August Wilhelm von Hofmann was the first to make stick-and-ball molecular models, which he used in lecture at the Royal Institution of Great Britain, such as methane shown below:

The basis of this model followed the earlier 1855 suggestion by his colleague William Odling that carbon is tetravalent. Hofmann's color scheme, to note, is still used to this day: nitrogen = blue, oxygen = red, chlorine = green, sulfur = yellow, hydrogen = white.[16] The deficiencies in Hofmann's model were essentially geometric: carbon bonding was shown as planar, rather than tetrahedral, and the atoms were out of proportion, e.g. carbon was smaller in size than the hydrogen.
In 1864, Scottish organic chemist Alexander Crum Brown began to draw pictures of molecules, in which he enclosed the symbols for atoms in circles, and used broken lines to connect the atoms together in a way that satisfied each atom's valence.
The year 1873, by many accounts, was a seminal point in the history of the development of the concept of the "molecule". In this year, the renowned Scottish physicist James Clerk Maxwell published his famous thirteen page article 'Molecules' in the September issue of Nature.[17] In the opening section to this article, Maxwell clearly states:
An atom is a body which cannot be cut in two; a molecule is the smallest possible portion of a particular substance.
After speaking about the atomic theory of Democritus, Maxwell goes on to tell us that the word 'molecule' is a modern word. He states, "it does not occur in Johnson's Dictionary. The ideas it embodies are those belonging to modern chemistry." We are told that an 'atom' is a material point, invested and surrounded by 'potential forces' and that when 'flying molecules' strike against a solid body in constant succession it causes what is called pressure of air and other gases. At this point, however, Maxwell notes that no one has ever seen or handled a molecule.
In 1874, Jacobus Henricus van 't Hoff and Joseph Achille Le Bel independently proposed that the phenomenon of optical activity could be explained by assuming that the chemical bonds between carbon atoms and their neighbors were directed towards the corners of a regular tetrahedron. This led to a better understanding of the three-dimensional nature of molecules.
Emil Fischer developed the Fischer projection technique for viewing 3-D molecules on a 2-D sheet of paper:
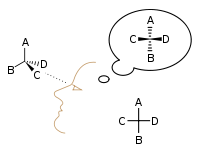
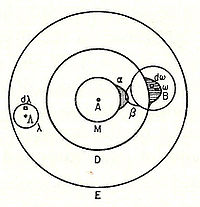
In 1898, Ludwig Boltzmann, in his Lectures on Gas Theory, used the theory of valence to explain the phenomenon of gas phase molecular dissociation, and in doing so drew one of the first rudimentary yet detailed atomic orbital overlap drawings. Noting first the known fact that molecular iodine vapor dissociates into atoms at higher temperatures, Boltzmann states that we must explain the existence of molecules composed of two atoms, the 「double atom」 as Boltzmann calls it, by an attractive force acting between the two atoms. Boltzmann states that this chemical attraction, owing to certain facts of chemical valence, must be associated with a relatively small region on the surface of the atom called the sensitive region.
Boltzmann states that this "sensitive region" will lie on the surface of the atom, or may partially lie inside the atom, and will firmly be connected to it. Specifically, he states 「only when two atoms are situated so that their sensitive regions are in contact, or partly overlap, will there be a chemical attraction between them. We then say that they are chemically bound to each other.」 This picture is detailed below, showing the α-sensitive region of atom-A overlapping with the β-sensitive region of atom-B:[18]
20世紀[編輯]
In the early 20th century, the American chemist Gilbert N. Lewis began to use dots in lecture, while teaching undergraduates at Harvard, to represent the electrons around atoms. His students favored these drawings, which stimulated him in this direction. From these lectures, Lewis noted that elements with a certain number of electrons seemed to have a special stability. This phenomenon was pointed out by the German chemist Richard Abegg in 1904, to which Lewis referred to as "Abegg's law of valence" (now generally known as Abegg's rule). To Lewis it appeared that once a core of eight electrons has formed around a nucleus, the layer is filled, and a new layer is started. Lewis also noted that various ions with eight electrons also seemed to have a special stability. On these views, he proposed the rule of eight or octet rule: Ions or atoms with a filled layer of eight electrons have a special stability.[19]
Moreover, noting that a cube has eight corners Lewis envisioned an atom as having eight sides available for electrons, like the corner of a cube. Subsequently, in 1902 he devised a conception in which cubic atoms can bond on their sides to form cubic-structured molecules.
In other words, electron-pair bonds are formed when two atoms share an edge, as in structure C below. This results in the sharing of two electrons. Similarly, charged ionic-bonds are formed by the transfer of an electron from one cube to another, without sharing an edge A. An intermediate state B where only one corner is shared was also postulated by Lewis.

Hence, double bonds are formed by sharing a face between two cubic atoms. This results in the sharing of four electrons.
In 1913, while working as the chair of the department of chemistry at the University of California, Berkeley, Lewis read a preliminary outline of paper by an English graduate student, Alfred Lauck Parson, who was visiting Berkeley for a year. In this paper, Parson suggested that the electron is not merely an electric charge but is also a small magnet (or "magneton" as he called it) and furthermore that a chemical bond results from two electrons being shared between two atoms.[20] This, according to Lewis, meant that bonding occurred when two electrons formed a shared edge between two complete cubes.
On these views, in his famous 1916 article The Atom and the Molecule, Lewis introduced the 「Lewis structure」 to represent atoms and molecules, where dots represent electrons and lines represent covalent bonds. In this article, he developed the concept of the electron-pair bond, in which two atoms may share one to six electrons, thus forming the single electron bond, a single bond, a double bond, or a triple bond.

路易斯認為:一個電子可能會屬於兩個不同原子,且不能說這個電子是任何一個原子獨佔的。
Moreover, he proposed that an atom tended to form an ion by gaining or losing the number of electrons needed to complete a cube. Thus, Lewis structures show each atom in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another; occasionally, pairs of dots are used instead of lines. Excess electrons that form lone pairs are represented as pair of dots, and are placed next to the atoms on which they reside:

To summarize his views on his new bonding model, Lewis states:[21]
Two atoms may conform to the rule of eight, or the octet rule, not only by the transfer of electrons from one atom to another, but also by sharing one or more pairs of electrons...Two electrons thus coupled together, when lying between two atomic centers, and held jointly in the shells of the two atoms, I have considered to be the chemical bond. We thus have a concrete picture of that physical entity, that "hook and eye" which is part of the creed of the organic chemist.
The following year, in 1917, an unknown American undergraduate chemical engineer named Linus Pauling was learning the Dalton hook-and-eye bonding method at the Oregon Agricultural College, which was the vogue description of bonds between atoms at the time. Each atom had a certain number of hooks that allowed it to attach to other atoms, and a certain number of eyes that allowed other atoms to attach to it. A chemical bond resulted when a hook and eye connected. Pauling, however, wasn't satisfied with this archaic method and looked to the newly-emerging field of quantum physics for a new method.
In 1927, the physicists Fritz London and Walter Heitler applied the new quantum mechanics to the deal with the saturable, nondynamic forces of attraction and repulsion, i.e., exchange forces, of the hydrogen molecule. Their valence bond treatment of this problem, in their joint paper,[22] was a landmark in that it brought chemistry under quantum mechanics. Their work was an influence on Pauling, who had just received his doctorate and visited Heitler and London in Zürich on a Guggenheim Fellowship.
Subsequently, in 1931, building on the work of Heitler and London and on theories found in Lewis' famous article, Pauling published his ground-breaking article "The Nature of the Chemical Bond"[23] (see: manuscript) in which he used quantum mechanics to calculate properties and structures of molecules, such as angles between bonds and rotation about bonds. On these concepts, Pauling developed hybridization theory to account for bonds in molecules such as CH4, in which four sp³ hybridised orbitals are overlapped by hydrogen's 1s orbital, yielding four sigma (σ) bonds. The four bonds are of the same length and strength, which yields a molecular structure as shown below:
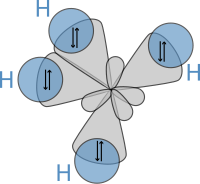

由於提出這些理論,鮑林榮獲1954年諾貝爾化學獎。值得一提的是,他是唯一一個贏得兩個非共享諾貝爾獎的人,他還在1962年因反對核武器的使用贏得諾貝爾和平獎。
1926年,法國物理學家讓·佩蘭因最終證明分子的存在而獲得諾貝爾物理學獎。他通過用包括液相的三種方法計算了阿伏伽德羅常數。第一他使用了一個藤黃肥皂樣乳液計算,然後通過做布朗運動的實驗計算,最後通過確認愛因斯坦提出的在液相中的粒子旋轉計算。[24]
1937年,化學家K.L. 沃爾夫引進了超分子的概念以描述通過氫鍵形成的乙酸二聚體。這個概念最後形成了研究由非共價鍵聚合分子的超分子化學學科。
1951年,物理學家歐文威廉·米勒發明了場離子顯微鏡並用它第一次看到原子。
在1999年,維也納大學的研究人員報告了對C60分子的波粒二象性研究的實驗結果。[25]塞林格等發佈的數據與C60分子的德布羅意波干擾是一致的。這個實驗大大提高了波粒二象性在宏觀上的適用範圍。[26]
21世紀[編輯]
在2009年,IBM的研究人員拍攝了分子的第一張真實照片。[27] 通過原子力顯微鏡,並五苯的每個原子和化學鍵都能被看到。
參見[編輯]
參考資料[編輯]
- ^ 《尚书·周书·洪范》 (簡體中文).
- ^ Russell, Bertrand. A History of Western Philosophy. Simon & Schuster. 2007: 41. ISBN 9781416554776 (英語).
- ^ Russell, Bertrand. A History of Western Philosophy. Simon & Schuster. 2007: 145. ISBN 9781416554776 (英語).
- ^ Pfeffer, Jeremy, I.; Nir, Shlomo. Modern Physics: An Introduction Text. World Scientific Publishing Company. 2001: 183. ISBN 1860942504.
- ^ See testimonia DK 68 A 80, DK 68 A 37 and DK 68 A 43. See also Cassirer, Ernst. An Essay on Man: an Introduction to the Philosophy of Human Culture. Doubleday & Co. 1953: 214. ISBN 0300000340. ASIN B0007EK5MM.
- ^ Waller, John. Leaps in the Dark: the Making of Scientific Reputations. Oxford University Press. 2004: 43. ISBN 0192804847.
- ^ Comments made by French chemist Guyton de Morveau in about 1772; as found in Kim’s 2003 Affinity That Elusive Dream – A Genealogy of the Chemical Revolution.
- ^ Leicester, Henry, M. The Historical Background of Chemistry. John Wiley & Sons. 1956: 112. ISBN 0486610535.
- ^ (a) Isaac Newton, (1704). Opticks. (pg. 389). New York: Dover.
(b) Bernard, Pullman; Reisinger, Axel, R. The Atom in the History of Human Thought. Oxford University Press. 2001: 139. ISBN 0195150406. - ^ Lemery, Nicolas. (1680). An Appendix to a Course of Chymistry. London, pgs 14-15.
- ^ Avogadro, Amedeo. Masses of the Elementary Molecules of Bodies. Journal de Physique. 1811, 73: 58–76.
- ^ Seymour H. Mauskopf. The Atomic Structural Theories of Ampère and Gaudin: Molecular Speculation and Avogadro's Hypothesis. Isis. 1969, 60 (1): 61–74. JSTOR 229022. doi:10.1086/350449.
- ^ Chemical Bonding Concepts – Oklahoma State University
- ^ Couper’s bond line drawings (1858) – Chemical Achievers
- ^ Bader, A. & Parker, L. (2001). "Joseph Loschmidt", Physics Today, Mar.
- ^ Ollis, W. D. Models and molecules. Proceedings of the Royal Institution of Great Britain. 1972, 45: 1–31. 已忽略未知參數
|author-separator=(幫助) - ^ Maxwell, James Clerk, "Molecules". Nature, September, 1873.
- ^ Boltzmann, Ludwig. Lectures on Gas Theory. Dover (reprint). 1898. ISBN 0486684555.
- ^ Cobb, Cathy. Creations of Fire - Chemistry's Lively History From Alchemy to the Atomic Age. Perseus Publishing. 1995. ISBN 0-7382-0594-X.
- ^ Parson, A.L. (1915). "A Magneton Theory of the Structure of the Atom". Smithsonian Publication 2371, Washington.
- ^ "Valence and The Structure of Atoms and Molecules", G. N. Lewis, American Chemical Society Monograph Series, page 79 and 81.
- ^ Heitler, Walter; London, Fritz. Wechselwirkung neutraler Atome und homöopolare Bindung nach der Quantenmechanik. Zeitschrift für Physik. 1927, 44: 455–472. Bibcode:1927ZPhy...44..455H. doi:10.1007/BF01397394.
- ^ Pauling, Linus. The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules. J. Am. Chem. Soc. 1931, 53: 1367–1400. doi:10.1021/ja01355a027. 已忽略未知參數
|author-separator=(幫助) - ^ Perrin, Jean, B. (1926). Discontinuous Structure of Matter, Nobel Lecture, December 11.(英文)
- ^ Arndt, M.; O. Nairz, J. Voss-Andreae, C. Keller, G. van der Zouw, A. Zeilinger. Wave-particle duality of C60 molecules. Nature. 1999, 401 (6754): 680–682. Bibcode:1999Natur.401..680A. PMID 18494170. doi:10.1038/44348 (英語). 已忽略未知參數
|month=(建議使用|date=) (幫助); - ^ Rae, A. I. M. Quantum physics: Waves, particles and fullerenes. Nature. 1999, 401 (6754): 651–653. Bibcode:1999Natur.401..651R. doi:10.1038/44294 (英語). 已忽略未知參數
|month=(建議使用|date=) (幫助) - ^ Single molecule's stunning image (英語).
擴展閱讀[編輯]
- Partington, J.R. A Short History of Chemistry. Dover Publications, Inc. 1989. ISBN 0-486-65977-1.(英文)
- Atkins, Peter. Atkins' Molecules, 2nd Ed. Cambridge University Press. 2003. ISBN 0-521-53536-0.(英文)
- Sargent, Ted. The Dance of Molecules - How Nanotechnology is Changing our Lives. Thunder's Mouth Press. 2006. ISBN 1-56025-809-8.(英文)
外部連結[編輯]
- 分子的幾何結構(英文) - 米德爾伯里學院
- 原子和分子(英文) - 麥克馬斯特大學
- 3D分子模型查看(英文) - The Wileys Family
- 本月分子(英文) - 英國布里斯托大學化學學院
- {{http://www.chem.pku.edu.cn/wuji/jiaoan/liuzhongfan/9.pdf 分子理論](簡體中文) - 北京大學化學與分子工程學院
分子種類[編輯]
分子定義[編輯]
文章[編輯]
- Molecules Used to Make Nano-sized Containers(英文) - Technology Research News (TRN) 通訊社
- Molecular Computer Processors(英文) - 惠普實驗室
