依布硒
| 依布硒 | |
|---|---|
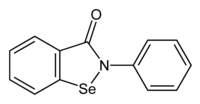
| |

| |

| |
| IUPAC名 2-Phenyl-1,2-benzoselenazol-3(2H)-one | |
| 識別 | |
| CAS編號 | 60940-34-3 |
| PubChem | 3194 |
| ChemSpider | 3082 |
| SMILES |
|
| InChI |
|
| InChIKey | DYEFUKCXAQOFHX-UHFFFAOYAZ |
| ChEBI | 77543 |
| 性質 | |
| 化學式 | C13H9NOSe |
| 摩爾質量 | 274.17666 g·mol⁻¹ |
| 若非註明,所有數據均出自標準狀態(25 ℃,100 kPa)下。 | |
依布硒(英語:Ebselen、也稱為PZ 51、DR3305和SPI-1005)是一種合成的有機硒藥物分子,具有抗炎、抗氧化和細胞保護活性。它充當穀胱甘肽過氧化物酶的模擬物,也可以與過氧亞硝酸鹽反應。[1]正在被研究作為再灌注損傷和中風、[2][3]聽覺損失和耳鳴[4][5]和雙向情緒障礙症[6][7]的可能治療藥物。
此外,依布硒也可以有效地對抗艱難擬梭菌感染[8]且它也被證明擁有對抗煙麴黴的抗真菌活性。[9]
依布硒是過氧化氫和氫過氧化物(包括膜結合磷脂和膽固醇酯氫過氧化物)的有效清除劑。幾種依布硒類似物已顯示在硫醇存在下清除過氧化氫。[10]
可能的抗COVID-19活性[編輯]
初步研究表明,依布硒在基於細胞的測定中表現出對COVID-19的抑制活性。[11][12][13]該效應歸因於通過與活性中心的半胱氨酸(Cys-145)的硫醇基團形成共價鍵對主要蛋白酶的不可逆抑制。[11]
合成[編輯]
通常,依布硒的特徵支架,苯並異硒唑酮環系統的合成,可以通過一級胺(RNH2)與2-(氯硒)苯甲酰氯(路線一)的反應、[14]苯甲酰苯胺的鄰位鋰化和氧化環化來實現(路線二)由溴化銅(CuBr2)介導,[15]或通過高效的銅催化的鄰鹵代苯甲酰胺的硒化/雜環化,這是Kumar等人開發的一種方法[16](路線三)。
參考文獻[編輯]
- ^ Schewe T. Molecular actions of ebselen--an antiinflammatory antioxidant. General Pharmacology. October 1995, 26 (6): 1153–69. PMID 7590103. doi:10.1016/0306-3623(95)00003-J.
- ^ Parnham M, Sies H. Ebselen: prospective therapy for cerebral ischaemia. Expert Opinion on Investigational Drugs. March 2000, 9 (3): 607–19. PMID 11060699. S2CID 42599736. doi:10.1517/13543784.9.3.607.
- ^ Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, Yasuhara H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke. January 1998, 29 (1): 12–7. PMID 9445321. doi:10.1161/01.STR.29.1.12
 .
.
- ^ Kil J, Pierce C, Tran H, Gu R, Lynch ED. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hearing Research. April 2007, 226 (1–2): 44–51. PMID 17030476. S2CID 39896274. doi:10.1016/j.heares.2006.08.006.
- ^ Kil, Jonathan; Harruff, E. Emily; Longenecker, Ryan J. Development of ebselen for the treatment of sensorineural hearing loss and tinnitus. Hearing Research (Elsevier BV). 2022, 413: 108209. ISSN 0378-5955. PMID 33678494. S2CID 231956488. doi:10.1016/j.heares.2021.108209.
- ^ Singh N, Halliday AC, Thomas JM, Kuznetsova OV, Baldwin R, Woon EC, et al. A safe lithium mimetic for bipolar disorder. Nature Communications. 2013, 4: 1332. Bibcode:2013NatCo...4.1332S. PMC 3605789
 . PMID 23299882. doi:10.1038/ncomms2320.
. PMID 23299882. doi:10.1038/ncomms2320.
- ^ New drug for bipolar disorder may offer fewer side effects. University of Oxford Latest News. 2013-01-08 [12 January 2013]. (原始內容存檔於2020-08-05).
- ^ Drug disarms deadly C. difficile bacteria without destroying healthy gut flora. Medical Express. [2022-10-22]. (原始內容存檔於2020-09-23).
- ^ Marshall AC, Kidd SE, Lamont-Friedrich SJ, Arentz G, Hoffmann P, Coad BR, Bruning JB. Structure, Mechanism, and Inhibition of Aspergillus fumigatus Thioredoxin Reductase. Antimicrobial Agents and Chemotherapy. March 2019, 63 (3): e02281–18. PMC 6395915
 . PMID 30642940. doi:10.1128/AAC.02281-18
. PMID 30642940. doi:10.1128/AAC.02281-18  .
.
- ^ Satheeshkumar K, Mugesh G. Synthesis and antioxidant activity of peptide-based ebselen analogues. Chemistry. April 2011, 17 (17): 4849–57. PMID 21400619. doi:10.1002/chem.201003417.
- ^ 11.0 11.1 Jin Z, Du X, Xu Y, Deng Y, Liu M, Zhao Y, et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. June 2020, 582 (7811): 289–293. Bibcode:2020Natur.582..289J. PMID 32272481. doi:10.1038/s41586-020-2223-y
 .
.
- ^ Weglarz-Tomczak E, Tomczak JM, Talma M, Burda-Grabowska M, Giurg M, Brul S. Identification of ebselen and its analogues as potent covalent inhibitors of papain-like protease from SARS-CoV-2. Scientific Reports. February 2021, 11 (1): 3640. Bibcode:2021NatSR..11.3640W. PMC 7878891
 . PMID 33574416. doi:10.1038/s41598-021-83229-6.
. PMID 33574416. doi:10.1038/s41598-021-83229-6.
- ^ Xiang R, Yu Z, Wang Y, Wang L, Huo S, Li Y, et al. Recent advances in developing small-molecule inhibitors against SARS-CoV-2. Acta Pharmaceutica Sinica. B. July 2021, 12 (4): 1591–1623. PMC 8260826
 . PMID 34249607. doi:10.1016/j.apsb.2021.06.016.
. PMID 34249607. doi:10.1016/j.apsb.2021.06.016.
- ^ Kamigata N, Iizuka H, Izuoka A, Kobayashi M. Photochemical Reaction of 2-Aryl-1, 2-benzisoselenazol-3 (2 H)-ones.. Bulletin of the Chemical Society of Japan. July 1986, 59 (7): 2179–83. doi:10.1246/bcsj.59.2179
 .
.
- ^ Engman L, Hallberg A. Expedient synthesis of ebselen and related compounds. The Journal of Organic Chemistry. 1989-06-01, 54 (12): 2964–2966. ISSN 0022-3263. doi:10.1021/jo00273a035.
- ^ Balkrishna SJ, Bhakuni BS, Chopra D, Kumar S. Cu-catalyzed efficient synthetic methodology for ebselen and related Se-N heterocycles. Organic Letters. December 2010, 12 (23): 5394–7. PMID 21053969. doi:10.1021/ol102027j.

