3C样蛋白酶
| SARS冠状病毒主蛋白酶 | |||||||
|---|---|---|---|---|---|---|---|
| |||||||
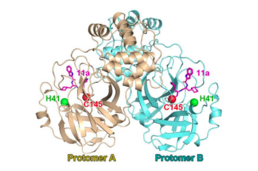
| SARS冠状病毒主蛋白酶二聚体与催化二联体(H41;C145)与共价肽模拟蛋白酶抑制剂(“11a”,洋红色)复合。从 PDB 6LZE.[1] | |||||||
| |||||||
| 识别码 | |||||||
| EC編號 | 3.4.22.69 | ||||||
| 数据库 | |||||||
| IntEnz | IntEnz浏览 | ||||||
| BRENDA | BRENDA入口 | ||||||
| ExPASy | NiceZyme浏览 | ||||||
| KEGG | KEGG入口 | ||||||
| MetaCyc | 代谢路径 | ||||||
| PRIAM | 概述 | ||||||
| PDB | RCSB PDB PDBj PDBe PDBsum | ||||||
| |||||||
| C30肽酶、冠状病毒内肽酶 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 鑑定 | |||||||||
| 標誌 | Peptidase_C30 | ||||||||
| Pfam | PF05409(旧版) | ||||||||
| InterPro | IPR008740 | ||||||||
| PROSITE | PS51442 | ||||||||
| MEROPS | C30 | ||||||||
| SCOP | d1q2wb1 / SUPFAM | ||||||||
| |||||||||
3C样蛋白酶(3CLpro,英语:3C-like protease)或称主蛋白酶(Mpro),前称C30内肽酶或3-糜蛋白酶样蛋白酶,[2]是在冠状病毒中发现的主要蛋白酶。它在11个保守位点切割冠状病毒多聚蛋白。它是一种半胱氨酸蛋白酶,也是蛋白酶PA家族的成员。它在其活性位点具有半胱氨酸-组氨酸催化二联体,并切Gln-(Ser/Ala/Gly)肽键。
酶学委员会将这个家族称为SARS冠状病毒主蛋白酶(Mpro; EC 3.4.22.69)。3C样蛋白酶对应了冠状病毒非结构蛋白5(nsp5)。通用名称中的“3C”是指3C蛋白酶(3Cpro),它是一种存在于微小核糖核酸病毒中的同源蛋白酶。
功能[编辑]
3C样蛋白酶能够催化裂解P1位谷氨酰胺和P1'位小氨基酸(丝氨酸、丙氨酸或甘氨酸)之间的肽键。例如,SARS 冠状病毒 3CLpro可以自我切割以下肽:[3][4][5]
蛋白酶在冠状病毒复制酶多蛋白的加工中很重要(UniProt P0C6U8)。它是冠状病毒中的主要蛋白酶,对应于非结构蛋白5 (nsp5)。[6]它在11个保守位点切割冠状病毒多蛋白。 3C样蛋白酶在其活性位点具有半胱氨酸-组氨酸催化二联体。[4]半胱氨酸的硫作为亲核试剂,组氨酸的咪唑环作为一般碱基。[7]
| 位置 | 基材偏好 |
|---|---|
| P5 | 无强烈的偏好 |
| P4 | 小的疏水残基 |
| P3 | 带正电荷的残基 |
| P2 | 高疏水性和无β-支链 |
| P1 | 谷氨酰胺 |
| P1' | 少量残留物 |
| P2' | 少量残留物 |
| P3' | 无强烈的偏好 |
命名法[编辑]
EC提供的替代名称包括3CLpro、3C样蛋白酶、冠状病毒3C样蛋白酶、Mpro、SARS 3C样蛋白酶、SARS冠状病毒3CL蛋白酶、SARS冠状病毒主肽酶、SARS冠状病毒主蛋白酶、SARS-CoV 3CLpro酶、SARS-CoV主蛋白酶、SARS-CoV Mpro和严重急性呼吸综合征冠状病毒主蛋白酶。
作为治疗靶点[编辑]

]
3C蛋白酶是冠状病毒感染的潜在药物靶标,因为它在处理从病毒RNA翻译的多聚蛋白中起重要作用。[11][12]未配位的3C蛋白酶及其与α-酮酰胺抑制剂的复合物的X射线结构[13]为设计用于治疗2型新冠病毒感染的α-酮酰胺抑制剂提供了基础。[14][15][16][17][18]
许多针对3CLpro和同源3Cpro的蛋白酶抑制剂正在开发中,包括3CLpro-1、GC376、芦平曲韦、卢夫特韦、奈玛特韦和AG7404。[19][20][21][22][1]静脉注射前体药物lufotrelvir于2020年9月进入临床试验。[23]口服活性的后续药物奈玛特韦作为与利托那韦的组合药物处于 II/III 期临床试验中,并于2021年11月公布了结果,包括在COVID-19症状发作后的三天内给予药物治疗可以减少89%的住院率。[24]一项包含2.35亿个分子的超大型虚拟筛选活动能够识别出一种针对几种冠状病毒主要蛋白酶的新型广谱抑制剂。[25]
2022年5月25日,英科智能通过人工智能来探索使用3C样蛋白酶抑制剂治疗COVID-19。临床前候选药物具有由人工智能设计的专门结构,被提名用于靶向3C样蛋白酶。它充当抑制剂并与2型新冠病毒和MERS病毒的3C样蛋白酶结合,使其失效并抑制病毒复制。临床前候选药物用于通过防止冠状病毒复制来治疗已经感染的人。[26]

其它3C(样)蛋白酶[编辑]
3C样蛋白酶广泛存在于(+)ssRNA病毒中。它们都是半胱氨酸蛋白酶,具有糜蛋白酶样折叠(PA族),使用催化二联体或三联体。它们在底物特异性和抑制剂有效性方面有一些普遍的相似之处。它们按序列相似性分为亚家族,对应于它们所在的病毒家族:[27]
- 微小核糖核酸病毒科具有3C蛋白酶 (EC 3.4.22.28; IPR000199; MEROPS C03)。这是最早研究的科。其他例子包括在脊髓灰质炎病毒和鼻病毒(两者都是肠道病毒属的成员)。
- 杯状病毒科有一个3C样蛋白酶(IPR001665; MEROPS C37),比如诺如病毒。
参见[编辑]
参考文献[编辑]
- ^ 1.0 1.1 1.2 Dai W, Zhang B, Jiang XM, Su H, Li J, Zhao Y, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. June 2020, 368 (6497): 1331–1335. Bibcode:2020Sci...368.1331D. PMC 7179937
 . PMID 32321856. doi:10.1126/science.abb4489
. PMID 32321856. doi:10.1126/science.abb4489  .
.
- ^ Ahmad, Bilal; Batool, Maria; Ain, Qurat ul; Kim, Moon Suk; Choi, Sangdun. Exploring the Binding Mechanism of PF-07321332 SARS-CoV-2 Protease Inhibitor through Molecular Dynamics and Binding Free Energy Simulations. International Journal of Molecular Sciences. 2021-08-24, 22 (17) [2022-09-16]. ISSN 1422-0067. PMC 8430524
 . PMID 34502033. doi:10.3390/ijms22179124. (原始内容存档于2021-10-06).
. PMID 34502033. doi:10.3390/ijms22179124. (原始内容存档于2021-10-06).
- ^ Goetz DH, Choe Y, Hansell E, Chen YT, McDowell M, Jonsson CB, Roush WR, McKerrow J, Craik CS. Substrate specificity profiling and identification of a new class of inhibitor for the major protease of the SARS coronavirus. Biochemistry. July 2007, 46 (30): 8744–52. PMID 17605471. doi:10.1021/bi0621415.
- ^ 4.0 4.1 Fan K, Wei P, Feng Q, Chen S, Huang C, Ma L, Lai B, Pei J, Liu Y, Chen J, Lai L. Biosynthesis, purification, and substrate specificity of severe acute respiratory syndrome coronavirus 3C-like proteinase. The Journal of Biological Chemistry. January 2004, 279 (3): 1637–42. PMC 7980035
 . PMID 14561748. doi:10.1074/jbc.m310875200
. PMID 14561748. doi:10.1074/jbc.m310875200  .
.
- ^ Akaji K, Konno H, Onozuka M, Makino A, Saito H, Nosaka K. Evaluation of peptide-aldehyde inhibitors using R188I mutant of SARS 3CL protease as a proteolysis-resistant mutant. Bioorganic & Medicinal Chemistry. November 2008, 16 (21): 9400–8. PMC 7126698
 . PMID 18845442. doi:10.1016/j.bmc.2008.09.057.
. PMID 18845442. doi:10.1016/j.bmc.2008.09.057.
- ^ Fehr AR, Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Maier HJ, Bickerton E, Britton P (编). Coronaviruses. Methods in Molecular Biology 1282. Springer. 2015: 1–23. ISBN 978-1-4939-2438-7. PMC 4369385
 . PMID 25720466. doi:10.1007/978-1-4939-2438-7_1.
. PMID 25720466. doi:10.1007/978-1-4939-2438-7_1. See section: Virion Structure.
- ^ Ryu YB, Park SJ, Kim YM, Lee JY, Seo WD, Chang JS, et al. SARS-CoV 3CLpro inhibitory effects of quinone-methide triterpenes from Tripterygium regelii. Bioorganic & Medicinal Chemistry Letters. March 2010, 20 (6): 1873–6. ISSN 0960-894X. PMC 7127101
 . PMID 20167482. doi:10.1016/j.bmcl.2010.01.152.
. PMID 20167482. doi:10.1016/j.bmcl.2010.01.152.
- ^ Chuck CP, Chow HF, Wan DC, Wong KB. Profiling of substrate specificities of 3C-like proteases from group 1, 2a, 2b, and 3 coronaviruses. PLoS One. 2011, 6 (11): e27228. PMC 3206940
 . PMID 22073294. doi:10.1371/journal.pone.0027228.
. PMID 22073294. doi:10.1371/journal.pone.0027228.
- ^ Vandyck K, Deval J. Considerations for the discovery and development of 3-chymotrypsin-like cysteine protease inhibitors targeting SARS-CoV-2 infection. Curr Opin Virol. August 2021, 49: 36–40. PMC 8075814
 . PMID 34029993. doi:10.1016/j.coviro.2021.04.006.
. PMID 34029993. doi:10.1016/j.coviro.2021.04.006.
- ^ Pfizer begins dosing in Phase II/III trial of antiviral drug for Covid-19.. Clinical Trials Arena. 2 September 2021.
- ^ Hilgenfeld R. From SARS to MERS: crystallographic studies on coronaviral proteases enable antiviral drug design. FEBS Journal. July 2014, 281 (18): 4085–4096. PMID 25039866. doi:10.1111/febs.12936.
- ^ Ullrich S, Nitsche C. The SARS-CoV-2 main protease as a drug target. Bioorganic & Medicinal Chemistry Letters. July 2020, 30 (17): 127377. PMID 32738988. doi:10.1016/j.bmcl.2020.127377.
- ^ Ocain TD, Rich DH. alpha-Keto amide inhibitors of aminopeptidases. Journal of Medicinal Chemistry. February 1992, 35 (3): 451–6. PMID 1738140. doi:10.1021/jm00081a005.
- ^ Anand K, Ziebuhr J, Wadhwani P, Mesters JR, Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. June 2003, 300 (5626): 1763–7. Bibcode:2003Sci...300.1763A. PMID 12746549. doi:10.1126/science.1085658
 .
.
- ^ Pacifico S, Ferretti V, Albanese V, Fantinati A, Gallerani E, Nicoli F, et al. Synthesis and Biological Activity of Peptide α-Ketoamide Derivatives as Proteasome Inhibitors. ACS Medicinal Chemistry Letters. July 2019, 10 (7): 1086–1092. PMC 6627721
 . PMID 31312413. doi:10.1021/acsmedchemlett.9b00233.
. PMID 31312413. doi:10.1021/acsmedchemlett.9b00233.
- ^ Kusov Y, Tan J, Alvarez E, Enjuanes L, Hilgenfeld R. A G-quadruplex-binding macrodomain within the "SARS-unique domain" is essential for the activity of the SARS-coronavirus replication-transcription complex. Virology. October 2015, 484: 313–22. PMC 4567502
 . PMID 26149721. doi:10.1016/j.virol.2015.06.016.
. PMID 26149721. doi:10.1016/j.virol.2015.06.016.
- ^ Zhang L, Lin D, Kusov Y, Nian Y, Ma Q, Wang J, et al. α-Ketoamides as Broad-Spectrum Inhibitors of Coronavirus and Enterovirus Replication: Structure-Based Design, Synthesis, and Activity Assessment. Journal of Medicinal Chemistry. February 2020, 63 (9): 4562–4578. PMC 7098070
 . PMID 32045235. doi:10.1021/acs.jmedchem.9b01828.
. PMID 32045235. doi:10.1021/acs.jmedchem.9b01828.
- ^ Zhang L, Lin D, Sun X, Curth U, Drosten C, Sauerhering L, et al. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science. March 2020, 368 (6489): 409–412. Bibcode:2020Sci...368..409Z. PMC 7164518
 . PMID 32198291. doi:10.1126/science.abb3405
. PMID 32198291. doi:10.1126/science.abb3405  .
.
- ^ Tian D, Liu Y, Liang C, Xin L, Xie X, Zhang D, Wan M, Li H, Fu X, Liu H, Cao W. An update review of emerging small-molecule therapeutic options for COVID-19. Biomedicine & Pharmacotherapy. May 2021, 137: 111313. PMC 7857046
 . PMID 33556871. doi:10.1016/j.biopha.2021.111313.
. PMID 33556871. doi:10.1016/j.biopha.2021.111313.
- ^ Morse JS, Lalonde T, Xu S, Liu WR. Learning from the Past: Possible Urgent Prevention and Treatment Options for Severe Acute Respiratory Infections Caused by 2019-nCoV. ChemBioChem. March 2020, 21 (5): 730–738. PMC 7162020
 . PMID 32022370. doi:10.1002/cbic.202000047.
. PMID 32022370. doi:10.1002/cbic.202000047.
- ^ Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Central Science. March 2020, 6 (3): 315–331. PMC 7094090
 . PMID 32226821. doi:10.1021/acscentsci.0c00272
. PMID 32226821. doi:10.1021/acscentsci.0c00272  .
.
- ^ Ramajayam R, Tan KP, Liang PH. Recent development of 3C and 3CL protease inhibitors for anti-coronavirus and anti-picornavirus drug discovery. Biochemical Society Transactions. October 2011, 39 (5): 1371–5. PMID 21936817. doi:10.1042/BST0391371
 .
.
- ^ First-In-Human Study To Evaluate Safety, Tolerability, And Pharmacokinetics Following Single Ascending And Multiple Ascending Doses of PF-07304814 In Hospitalized Participants With COVID-19.. Clinical Trials. 24 June 2021 [3 July 2021]. (原始内容存档于2021-11-05).
- ^ Pfizer's Novel COVID-19 Oral Antiviral Treatment Candidate Reduced Risk Of Hospitalization Or Death By 89% In Interim Analysis Of Phase 2/3 EPIC-HR Study. Pfizer Inc. 5 November 2021 [2022-09-16]. (原始内容存档于2021-11-16).
- ^ Luttens A, Gullberg H, Abdurakhmanov E, Vo DD, Akaberi D, Talibov VO, et al. Ultralarge Virtual Screening Identifies SARS-CoV-2 Main Protease Inhibitors with Broad-Spectrum Activity against Coronaviruses. J Am Chem Soc. February 2022, 144 (7): 2905–2920. ISSN 0002-7863. PMC 8848513
 . PMID 35142215. doi:10.1021/jacs.1c08402.
. PMID 35142215. doi:10.1021/jacs.1c08402.
- ^ AI-designed COVID-19 drug nominated for preclinical trials. www.theregister.com. [2022-05-26]. (原始内容存档于2022-12-01) (英语).
- ^ Kim Y, Lovell S, Tiew KC, Mandadapu SR, Alliston KR, Battaile KP, et al. Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. Journal of Virology. November 2012, 86 (21): 11754–62. PMC 3486288
 . PMID 22915796. doi:10.1128/JVI.01348-12.
. PMID 22915796. doi:10.1128/JVI.01348-12.
- ^ Ziebuhr J, Bayer S, Cowley JA, Gorbalenya AE. The 3C-like proteinase of an invertebrate nidovirus links coronavirus and potyvirus homologs. Journal of Virology. January 2003, 77 (2): 1415–26. PMC 140795
 . PMID 12502857. doi:10.1128/jvi.77.2.1415-1426.2003
. PMID 12502857. doi:10.1128/jvi.77.2.1415-1426.2003  .
.
| ||||||||||||||||||||||||||||||||
